This is the instrument used to measure the pH of a liquid solution or sample. Here you can find the best instruments on the market sold by Colaver.
.
pHmeter portable
.
There are portable phmeters the size of a pen and bench-top devices with different measurements. The portable ones are cheaper and can be used in the field but have a lower accuracy than the bench-top ones.
Portable instruments have many applications depending on the electrode used.
For soil
 When you have to work in the field, i.e. outdoors, you have to use instruments that are easy to carry, small and handy. But also reliable as a measurement.
When you have to work in the field, i.e. outdoors, you have to use instruments that are easy to carry, small and handy. But also reliable as a measurement.
Among the most field-proven research are pH analyses of:
.
In the context of soil analysis, battery life must be considered as a determining factor in fieldwork.
for Water Analysis
For example, when measuring pH values in a watercourse, it is good to be equipped with a phmeter that can take the measurement directly on site.
for Aquariums
 The aquarium trade is widespread. For good maintenance and to keep animal species with specific characteristics, one has to equip oneself. Enthusiasts know that water, for certain species, must have a very precise pH value.
The aquarium trade is widespread. For good maintenance and to keep animal species with specific characteristics, one has to equip oneself. Enthusiasts know that water, for certain species, must have a very precise pH value.
More generally, water analysis is frequently applied to:
- aquariums
- swimming pools
- sports facilities
- purification plants
- aqueducts
.
Food analysis
The food sector also requires maximum control. There are different types of probes, both suitable for liquids and for solid and semi-solid materials. With these, different types of food can be analysed.
In food analysis, those most frequently subjected to ph analysis are:
- milk
- cheese
- wine
- beer
- bread
- sweets
Milk analysis
 Milk pH measurement is useful in controlling the process, such as pasteurisation. However, it is also useful for comparing the different processes it undergoes, without having to carry out a specific chemical analysis.
Milk pH measurement is useful in controlling the process, such as pasteurisation. However, it is also useful for comparing the different processes it undergoes, without having to carry out a specific chemical analysis.
Cheese analysis
Nowadays, there is no such thing as an artisanal cheesemaker who measures nothing. Rightly so, pH measurement has become normal practice. To be able to carry out the analysis correctly, however, one must know all the stages of the biological and chemical evolution of milk: from coagulation to the finished cheese.
For oenology
First of all, the phmeter, with its specific electrode, is suitable for the wine industry. It is used to measure the acidity of wine. It is also used by wineries marketing wine. The so-called bottling stage. In addition to this, the instrument is used to measure the acidity of wine to certify quality.
 Phmeter wine to learn about microbiology.
Phmeter wine to learn about microbiology.
Must and wine are biologically living substances. In order to vinify and preserve them correctly, one must know their microbiological stability. First, however, a basic concept must be clarified. Ph is not total acidity. Total acidity is the sum of the acids present. The pH, on the other hand, is the true value that indicates acid strength. This value is used to determine whether a must or wine is chemically and microbiologically stable.
Beer analysis
.
The phmeter to be used in brewing must be able to operate at high temperatures. This is why it is equipped with a special electrode with a glass body that does not get damaged. Today, as craft brewing becomes more widespread, the right instruments must be used to ensure quality.
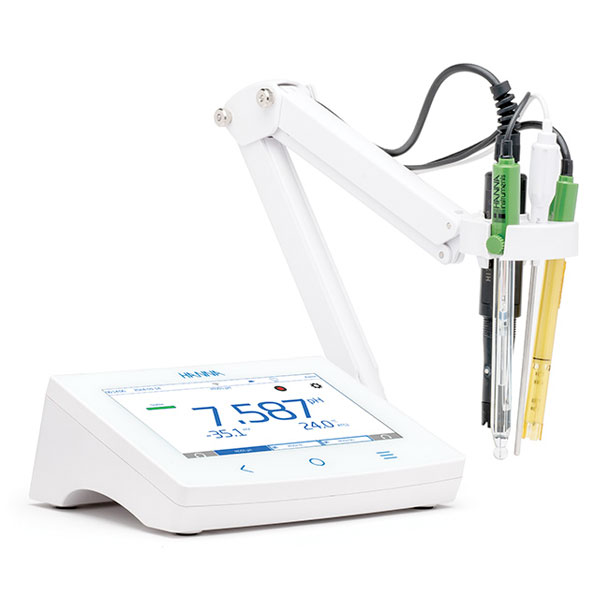
Laboratory pH meter
For stationary use in a laboratory, larger capacity instruments can be used. The term 'laboratory' refers to models capable of very precise measurements. They are usually equipped with a larger display and a pH electrode stand.
.
 Countertop phmeter
Countertop phmeter
In contrast to portable phmeters, laboratory phmeters are also called 'benchtop' phmeters. This is, of course, because they are larger in size and because they are equipped with many functions that smaller models do not have.
Among the most popular and popular on the market, Colaver sells the benchtop phmeter model Edge 2002-02 by Hanna.
Professional instrument
.
Although this is an unscientific definition, everyone realises a big difference. It is one thing to measure pH for craft activities carried out in one's spare time. It is a different thing to carry out analyses on products that are to be sold. For these purposes, the best instruments on the market must be used.
Multiparameter
Multiparameters are capable of measuring both pH and temperature. In addition to these, it also measures other chemical parameters such as Conductivity, Salinity, TDS. This can be possible by connecting several sensors. Multi-parameter instruments can be either portable or bench-top.
This type of device is widely used in the environmental analysis sector.
Digital pH meter = analytical measurement
The digital pH meter, like almost all equipment produced in recent decades, is an electronic instrument. The technology allows a very high level of accuracy and reading the values on a display is very convenient. I don't think anyone remembers the first post-war phmeters anymore, which were not yet digital.
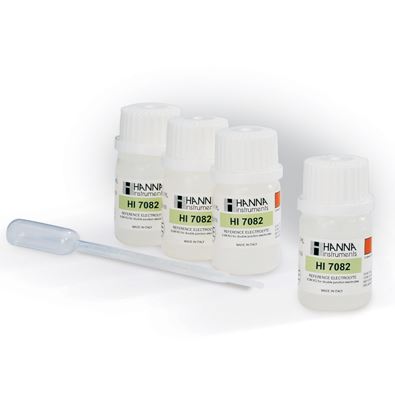
PH Solutions
Buffer solutions, in any case, are a liquid that resists pH change by moderate additions of acids or bases. And this applies to both old instruments and the digital phmeter.
Functioning
The pH meter works by means of a meter. Especially for industrial phmeters and those bench-mounted, the meter is like a voltmeter that shows results in pH units and not in volts. Due to the high electrical resistance of glass electrodes, the impedance must be very high. Approximately 20 to 1000 MΩ. The electrical potential of the probe must be amplified about 17 times and converted into pH units. To bring the signal within the pH scale, the result must be shifted by 7 units. At pH 7, the probe does not signal electrical potential. pH values greater than 7 are alkaline while those less than 7 are acidic.
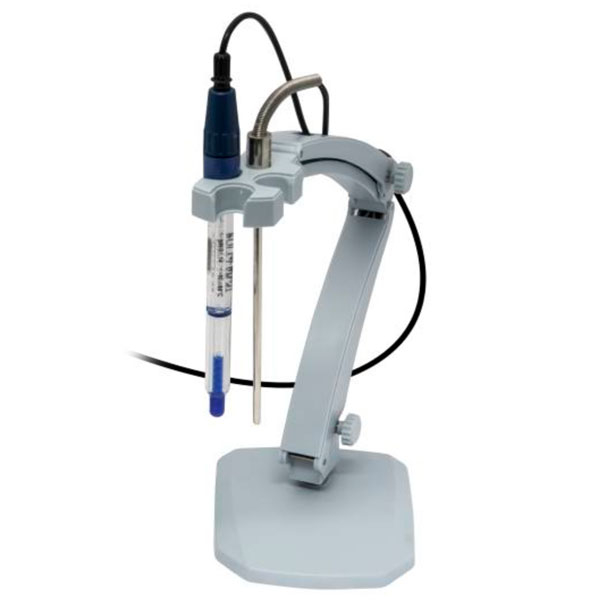
 Electrode and Probe
Electrode and Probe
A
pHmeter works with a probe consisting of an electrode called a 'glass' electrode, which collects data and sends it to the device. This shows the values on an electronic display.
PH measurement
.
The probe for measuring pH is an electrode that measures the difference in electrical potential on two sides of a glass membrane at the end of the electrode. The principle is based on the difference between the concentrations of hydrogen ions inside and outside the membrane. A pH unit produces a potential difference of approximately 0.059 V.
Temperature measurement
.
Sometimes, in addition to the pH value, the temperature is also measured with a specific probe, to correct the electrode value in relation to its temperature.
Calibration

The purpose of calibration is to align the result of the instrument with that expected from the reading of the standard solutions from the pH used as a reference. It is usually carried out with two or three standard buffer solutions. To be sure of the result, the instrument should be calibrated each time it is used. With modern equipment, however, the calibration remains stable for some time. There is either two-point or three-point calibration. For those who use the instrument daily, calibration is recommended at least every 20 measurements.
Price
When choosing an instrument for such important work, one should not only look at the price. Unfortunately, the scarcity of resources means that the laboratory budget must be used very carefully. This is why manufacturers have different price ranges for the instruments they sell.
We recommend considering these factors before a purchase:
- precision
- durability
- autonomy
- price
Hanna®hmeter
Among the most popular in Europe, the company
Hanna Instruments® offers many phmeter models. Also very popular are the
Phmometers XS®,
Mettler Toledo® instruments
.
This manufacturer is in a higher price bracket with very good quality instruments.
.



 When you have to work in the field, i.e. outdoors, you have to use instruments that are easy to carry, small and handy. But also reliable as a measurement.
When you have to work in the field, i.e. outdoors, you have to use instruments that are easy to carry, small and handy. But also reliable as a measurement. The aquarium trade is widespread. For good maintenance and to keep animal species with specific characteristics, one has to equip oneself. Enthusiasts know that water, for certain species, must have a very precise pH value.
The aquarium trade is widespread. For good maintenance and to keep animal species with specific characteristics, one has to equip oneself. Enthusiasts know that water, for certain species, must have a very precise pH value. Milk pH measurement is useful in controlling the process, such as pasteurisation. However, it is also useful for comparing the different processes it undergoes, without having to carry out a specific chemical analysis.
Milk pH measurement is useful in controlling the process, such as pasteurisation. However, it is also useful for comparing the different processes it undergoes, without having to carry out a specific chemical analysis. Phmeter wine to learn about microbiology.
Phmeter wine to learn about microbiology. Countertop phmeter
Countertop phmeter Electrode and Probe
Electrode and Probe The purpose of calibration is to align the result of the instrument with that expected from the reading of the standard solutions from the pH used as a reference. It is usually carried out with two or three standard buffer solutions. To be sure of the result, the instrument should be calibrated each time it is used. With modern equipment, however, the calibration remains stable for some time. There is either two-point or three-point calibration. For those who use the instrument daily, calibration is recommended at least every 20 measurements.
The purpose of calibration is to align the result of the instrument with that expected from the reading of the standard solutions from the pH used as a reference. It is usually carried out with two or three standard buffer solutions. To be sure of the result, the instrument should be calibrated each time it is used. With modern equipment, however, the calibration remains stable for some time. There is either two-point or three-point calibration. For those who use the instrument daily, calibration is recommended at least every 20 measurements.