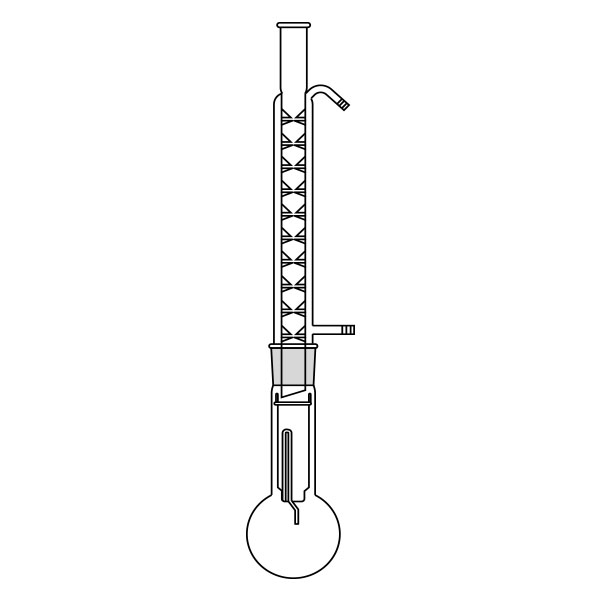
An extractor is a laboratory apparatus used for the extraction process. The most common model is the Soxhlet extractor, named after its inventor Franz von Soxhlet. Its original use was limited to the extraction of lipids from a solid material, using ethyl ether as a solvent. Today, it is commonly used, in place of filtration, for the extraction of a compound that has limited solubility in a solvent, and whose impurities are insoluble in that solvent.
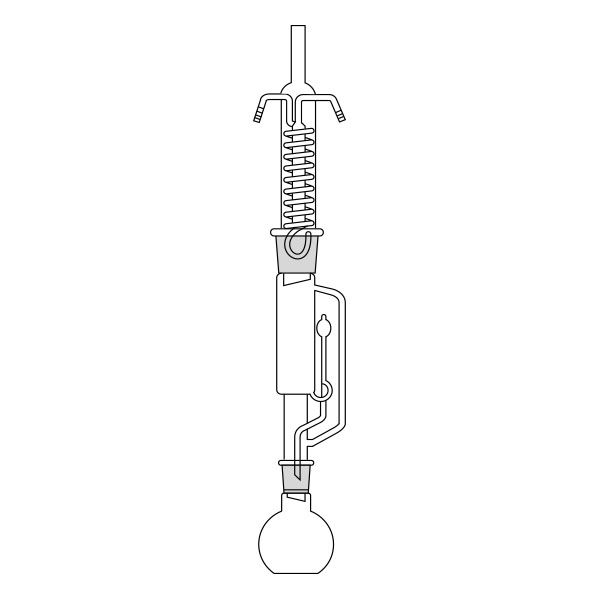
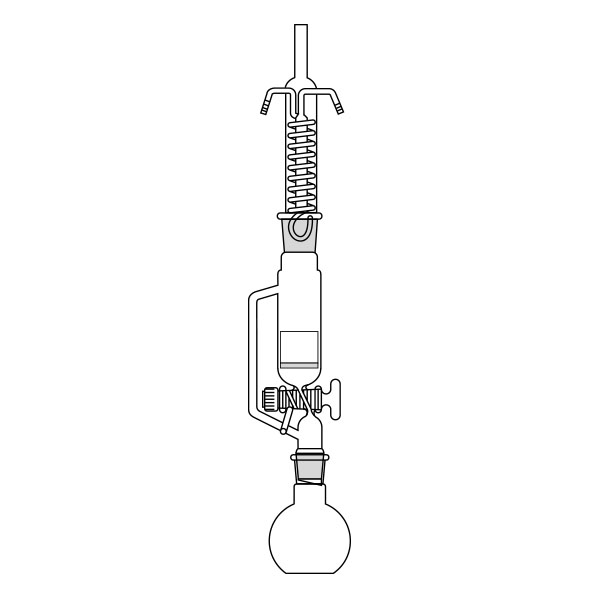
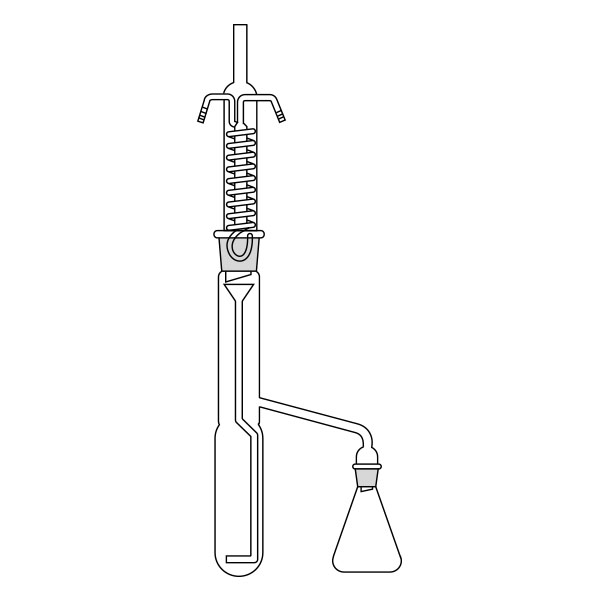
The Soxhlet extractor develops vertically, and consists of three main sections: a ground-neck flask, an extractor, and a condenser.
- Frosted neck balloon. It consists of the lower section of the three that make up the apparatus and serves as a container and reboiler for the solvent.
- The middle section, which is the lower section of the three that make up the apparatus, serves as a container and reboiler for the solvent.
- Middle section, in which the thimble filter is placed. The material to be extracted is placed in the extraction chamber of the thimble filter. The extractor is divided into two further overlapping chambers, which communicate with each other via two connectors. The lower chamber is connected to the flask, while the neck of the middle chamber is connected to a condenser.
The lower chamber is connected to the flask, while the neck of the middle chamber is connected to a condenser.
- Upper section of the extractor. The condenser allows the solvent vapours to condense. They then flow from the condenser into the extraction chamber, where the solid material is located.
The upper section of the extractor is connected to the flask.
Extraction process
The material to be extracted is placed in the extraction chamber, and the solvent in the flask, which is brought to the boil. Next, the solvent passes through the extractor and condenser, and finally reaches the extraction chamber as a condensate. In the extraction chamber, it is loaded with solute, which it carries with it through the filter paper. It is then tapped through a side duct, where solute accumulates at the bottom and solvent at the top. Subsequently, the solvent is recirculated to the flask at the bottom of the extractor. Due to the fact that the solvent is continuously recycled during the extraction process, the Soxhlet extractor has the advantage that less solvent is used.
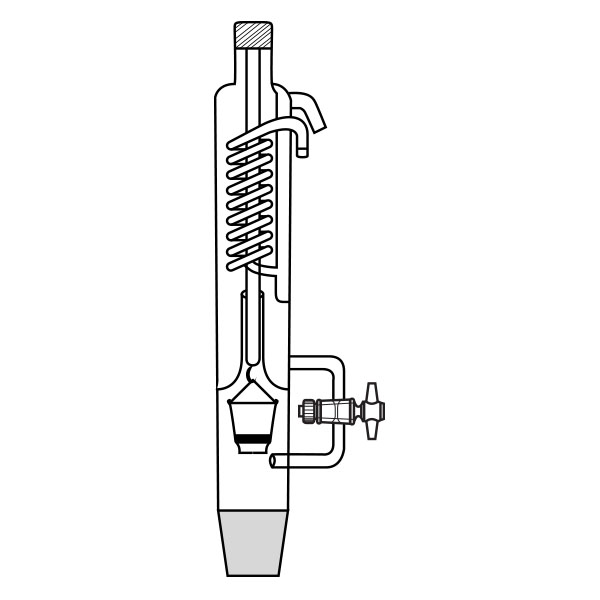
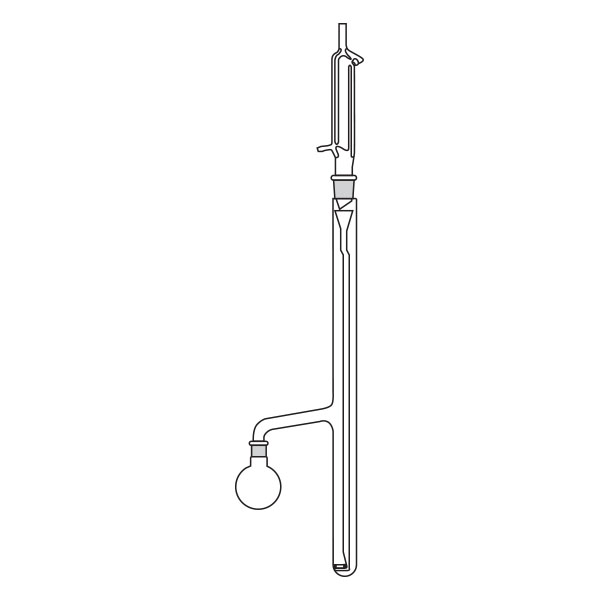
Kumagawa extractors
An alternative model of extractor is the Kumagawa extractor. It differs from Soxhlet's extractor in that the thimble filter is directly suspended in the solvent flask. It is surrounded by the hot solvent vapours and, consequently, maintained at a higher temperature than Soxhlet's extractor. This characteristic makes the Kumagawa extractor more suitable for the extraction of compounds with a high melting point, such as, for example, bitumen.