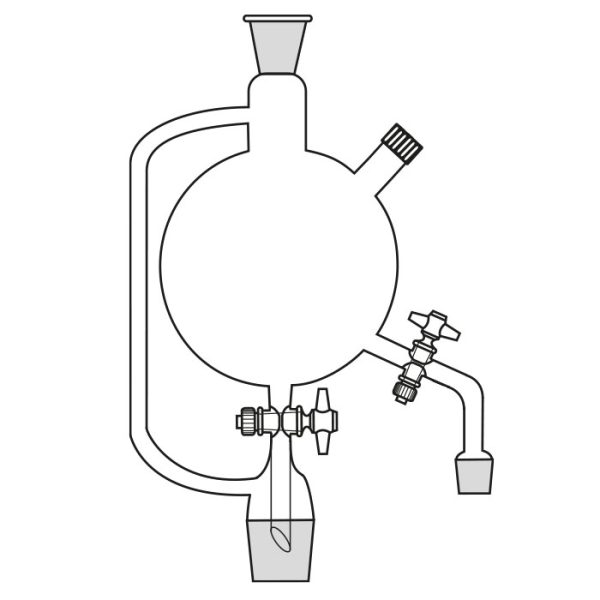
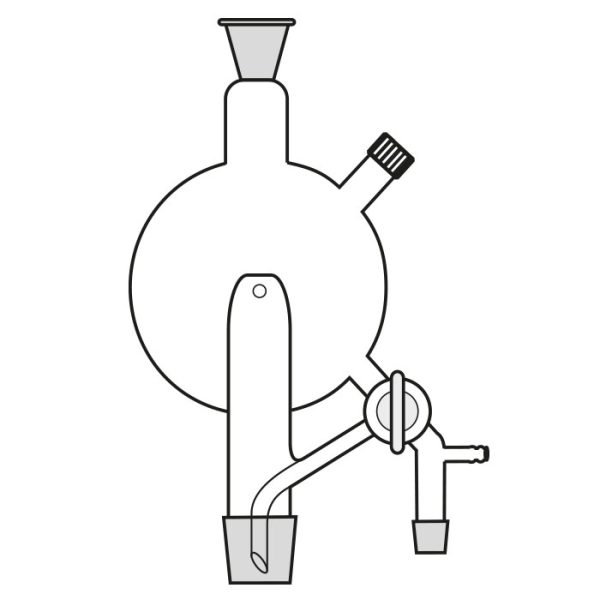
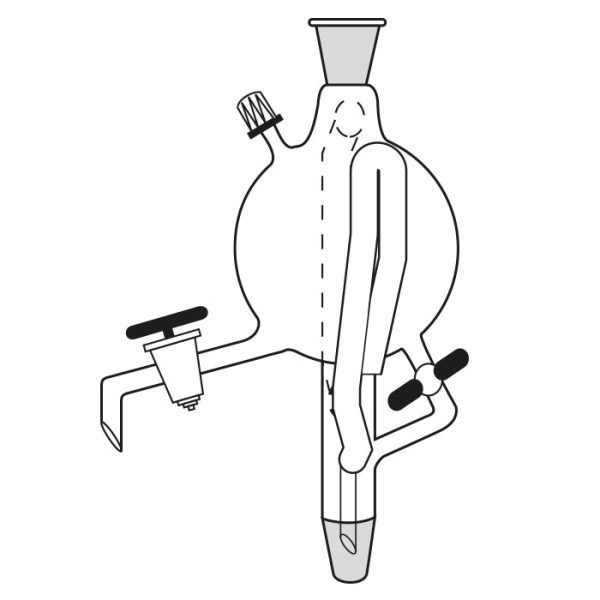
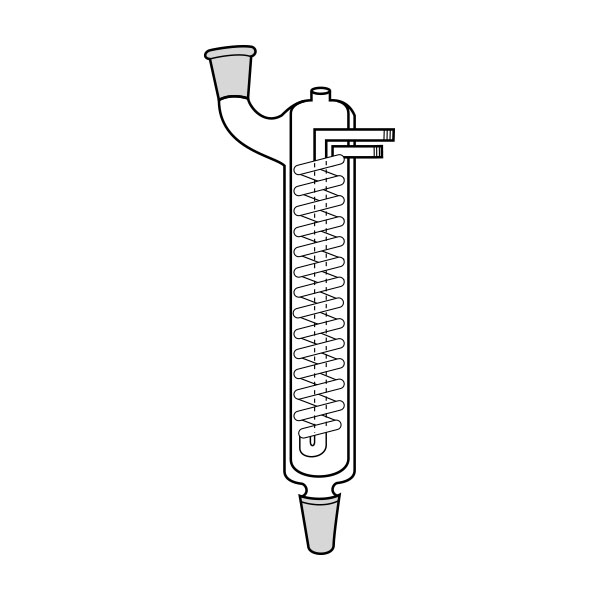
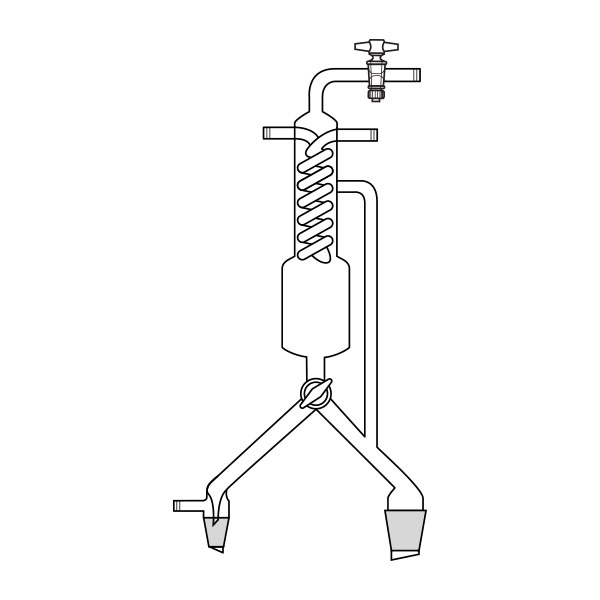
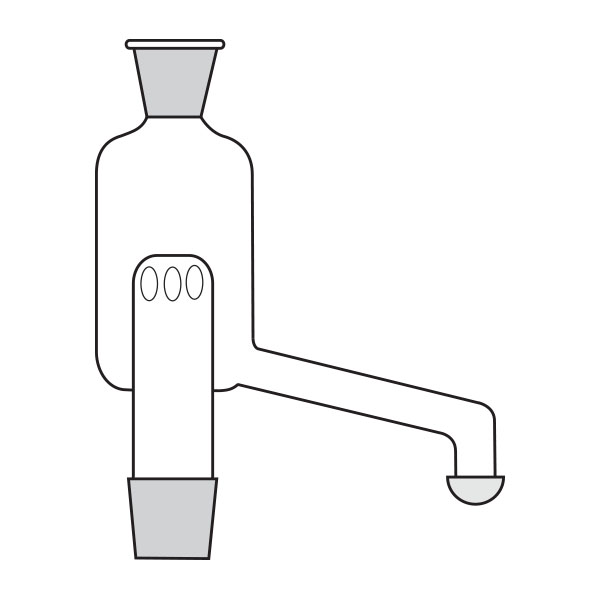
When the compounds to be distilled have very high boiling points, vacuum distillation is used. In the laboratory, it is well known that the boiling temperature of a substance is related to atmospheric pressure. By decreasing the pressure, the boiling temperature also decreases. With a vacuum pump, we can decrease the pressure within the system. It is still necessary to know the boiling temperature of the substance to be distilled at the new pressure.
 There is a tool, a graph called a nomograph, with which the new boiling temperature can be calculated. This will be valid for a different pressure value, comparing the boiling temperature of a compound at a certain pressure value. Before proceeding with the actual distillation, however, it is necessary to consider the pressure to be achieved by the vacuum pump we intend to use. Then we must calculate, through the use of the nomograph, the boiling point of the substance to be distilled at that particular pressure value.
There is a tool, a graph called a nomograph, with which the new boiling temperature can be calculated. This will be valid for a different pressure value, comparing the boiling temperature of a compound at a certain pressure value. Before proceeding with the actual distillation, however, it is necessary to consider the pressure to be achieved by the vacuum pump we intend to use. Then we must calculate, through the use of the nomograph, the boiling point of the substance to be distilled at that particular pressure value.
 Practice advises, in the case of vacuum distillation, to start heating only after applying vacuum. Heating before starting the pump would risk triggering violent boiling processes.
Practice advises, in the case of vacuum distillation, to start heating only after applying vacuum. Heating before starting the pump would risk triggering violent boiling processes.



 There is a tool, a graph called a nomograph, with which the new boiling temperature can be calculated. This will be valid for a different pressure value, comparing the boiling temperature of a compound at a certain pressure value. Before proceeding with the actual distillation, however, it is necessary to consider the pressure to be achieved by the vacuum pump we intend to use. Then we must calculate, through the use of the nomograph, the boiling point of the substance to be distilled at that particular pressure value.
There is a tool, a graph called a nomograph, with which the new boiling temperature can be calculated. This will be valid for a different pressure value, comparing the boiling temperature of a compound at a certain pressure value. Before proceeding with the actual distillation, however, it is necessary to consider the pressure to be achieved by the vacuum pump we intend to use. Then we must calculate, through the use of the nomograph, the boiling point of the substance to be distilled at that particular pressure value. Practice advises, in the case of vacuum distillation, to start heating only after applying vacuum. Heating before starting the pump would risk triggering violent boiling processes.
Practice advises, in the case of vacuum distillation, to start heating only after applying vacuum. Heating before starting the pump would risk triggering violent boiling processes.