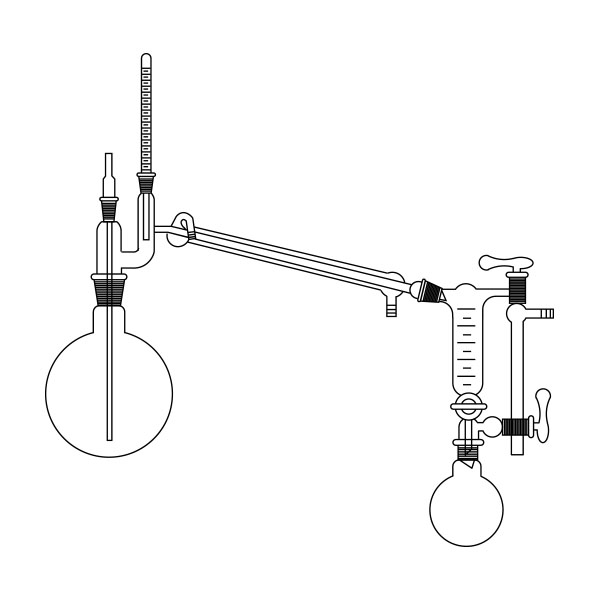
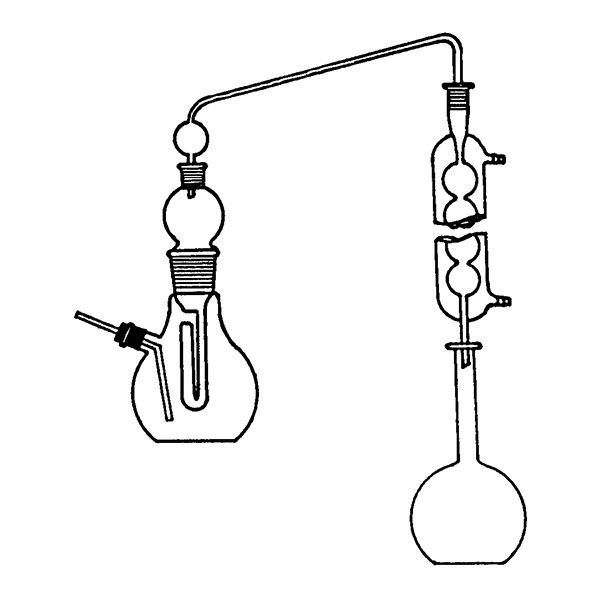
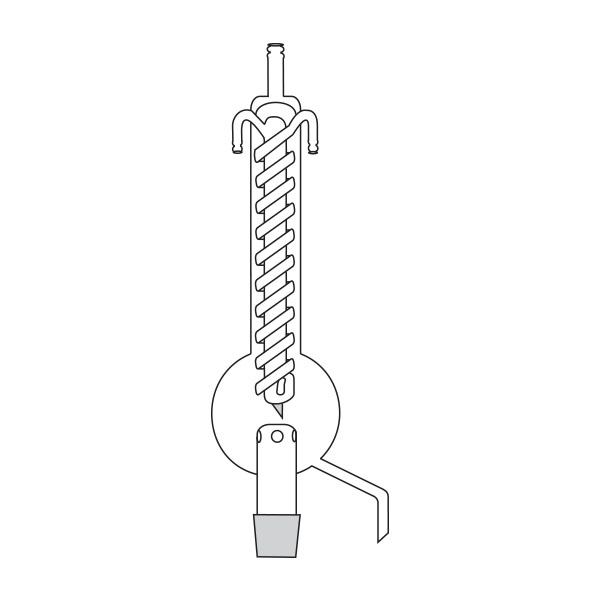
The laboratory distiller is mainly used to separate substances during research. To separate, it is necessary to understand which elements make up the solution. In laboratory distillers, once you know what differentiates substances, you can exploit this difference to perform the separation.
Separating two substances is not only done with the laboratory distiller, but with this one exploits the principle of splitting. In practice, you use the different boiling points of the substances you want to separate.
The different boiling points of the substances you want to separate.
There are usually different compounds in the solution, each characterised by a specific boiling point. Different from each other. By taking advantage of this, it is possible to bring the first component to the boil, letting it all evaporate. Once this is done, we can then reach the boiling point of the second component and evaporate that too. And there we have separated the two components of the mixture.
.
But let's take a closer look at what this technique consists of and what tools are needed to apply it.
Laboratory distiller to know the boiling point
.
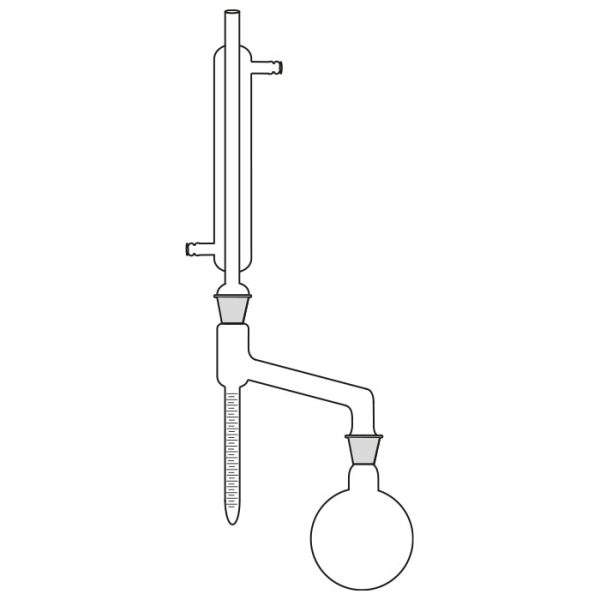
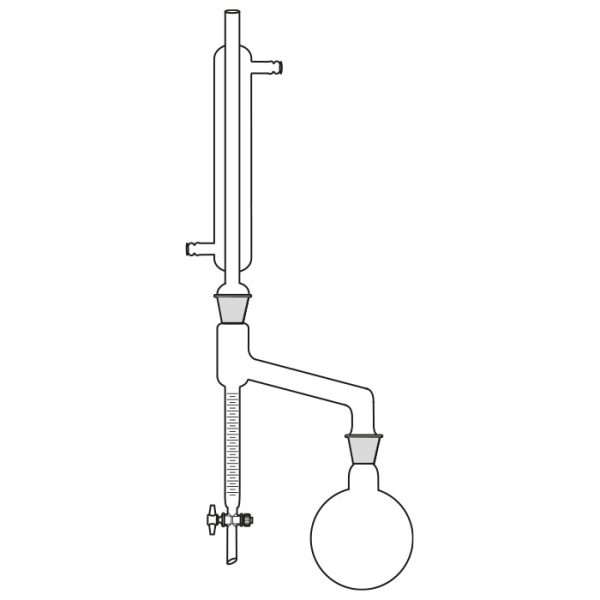
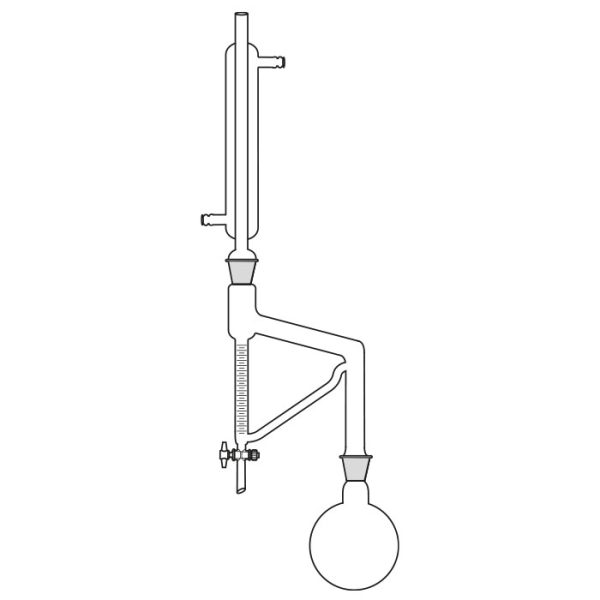
First, a glass flask must be used to contain the mixture of compounds we want to separate. This flask will be slowly and gradually heated. A special glass tube is connected to the flask. Vapours are conveyed into this. When the boiling point of the 'lowest boiling' component of the compound is reached. This tube is also called a coolant.

A thermometer is connected to the glass tube. The function of the instrument is to accurately indicate the temperature of the vapours reaching the bulb.
The temperature measured is the temperature of the vapours reaching the bulb.
The temperature measured is that of the vapours reaching almost to the top of the glass column. From the accuracy of this temperature we can get a relatively safe indication of the substance we are distilling. Obviously we must first know its boiling point.
The temperature inside the flask will be different. This measurement will not be useful from the point of view of separating the components of the mixture.
.
Constant temperature in the laboratory distiller
.
Constancy of temperature is another important point. It is essential to know the temperature of the vapours at the top of the glass column. This must remain uniform throughout the entire time the distillation is carried out.
This is a basic element of the principle of thermodynamics where we speak of latent heat of evaporation.
Latent heat refers to the amount of energy required for the transition from liquid to vapour to take place. We therefore speak of latent heat of evaporation.
In theory, it means that as long as a specific component of the mixture is evaporating, the temperature remains constant. This happens even if you continue heating. The excess heat is consumed in order to make the change of state take place. In this way, it does not increase the temperature of the system.
.
This is why measuring the vapour temperature at the top of the glass column indicates what is evaporating. It also indicates when the distillation of the first component finishes. In fact, according to this principle, as soon as the evaporation of the first compound in the mixture is finished, a rapid increase in temperature will be observed when heating continues.
Use of the laboratory distiller
In the distillation process, the distillation head and tail are discarded of all the material collected. These are the first and last drops of the distilled fraction. They are the ones most likely to contain a higher degree of impurities.
.
During distillation, the temperature remains constant. However, the first distilled drops may contain the presence of small amounts of 'low boiling' products. The impurities were carried away by the vapours of the component present in greater quantity.
The impurities were carried away by the vapours of the component present in greater quantity.
In contrast, at the end of the distillation it is possible that small quantities of the product of the first substance may mix with the product of the second substance and thus pollute the distillate.
For these reasons, to maintain the purity of the separation, this prior selection is recommended.
In this way, we have only discussed the basic principle of distillation. Many variations have been grafted onto this to be applied to the different mixtures that one wishes to separate or to the different objectives that one sets oneself in the laboratory.
The use of the laboratory distiller is based on exploiting the discriminating property of the different boiling points of the substances we wish to separate.
All of the many 'variations on the theme' are each more effective than another depending on the objective to be achieved and the characteristics of the mixture to be separated.
Each of these objectives has specific tools to be pursued, and from the experts at Colaver you will receive all the necessary guidance and advice on how to choose them and set up the equipment in your laboratory.