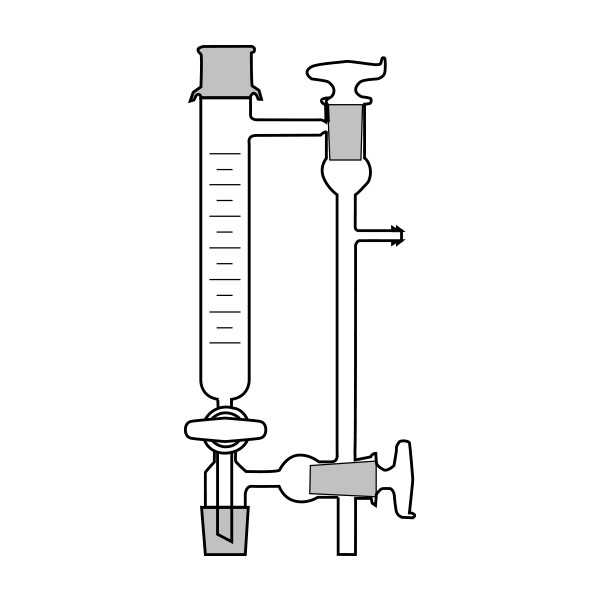
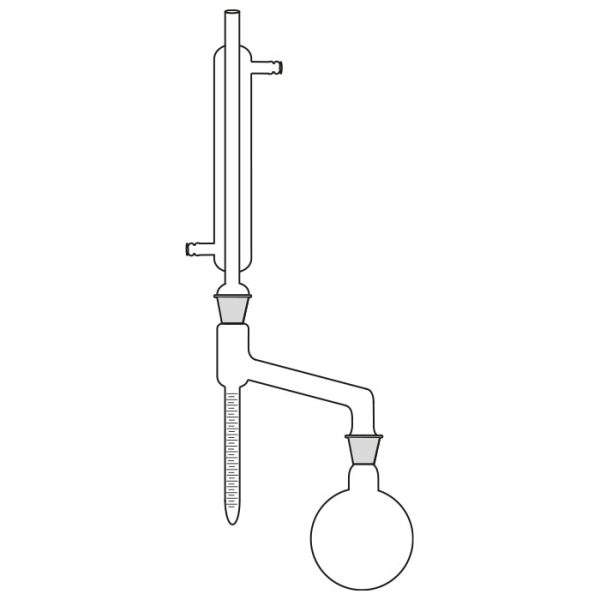
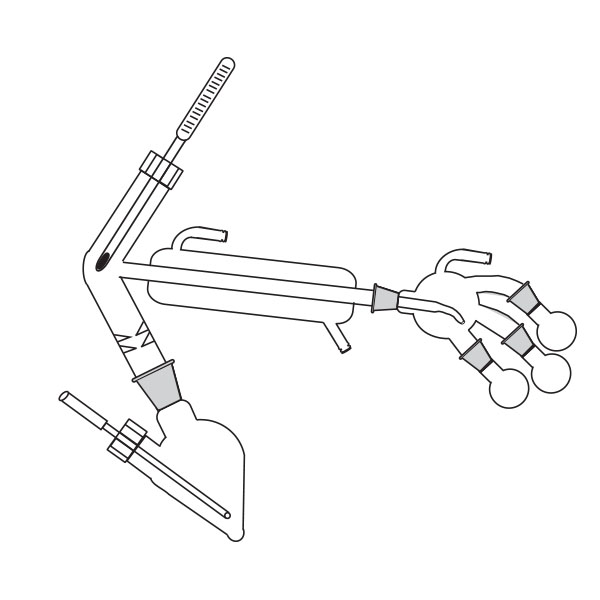
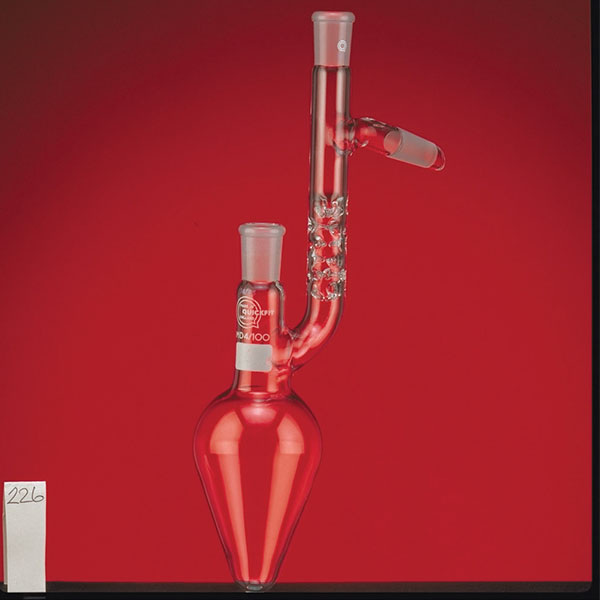
The distillation is a process of separating the components of a substance from a liquid mixture through evaporation and condensation. The chemical principle on which it is based is the different volatility of the components, i.e. the tendency of a specific substance to evaporate.
It can result in complete separation or partial separation. In the first case, the result is the different components pure, not mixed with other substances. In the second result you get a change in concentration of one of the components in the mixture.
.
Distillation applications
 The distillation is a technique known since antiquity and is carried out using the distiller. There are documents indicating its use by Egyptian peoples in 4000 BC, for the distillation of alcoholic beverages. Subsequently, the use of the distiller in the laboratory was already known in the Middle Ages.
The distillation is a technique known since antiquity and is carried out using the distiller. There are documents indicating its use by Egyptian peoples in 4000 BC, for the distillation of alcoholic beverages. Subsequently, the use of the distiller in the laboratory was already known in the Middle Ages.
Today, it is still used in various fields. For example, the distiller is used both in the chemical laboratory and in industry. In addition, distillation is still very common in food applications or for the distillation of wine. The very name of alcoholic spirits, such as grappa or cognac, suggest its use.
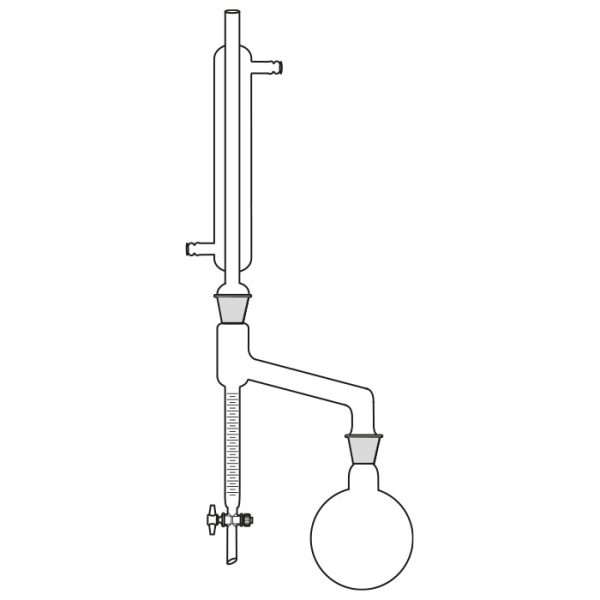
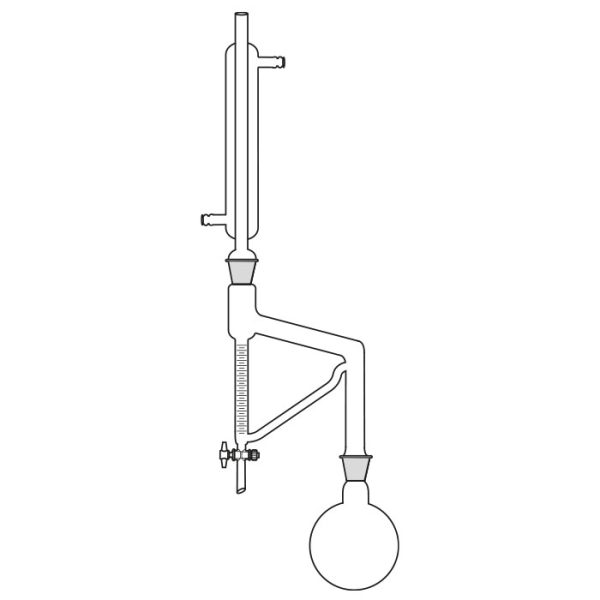
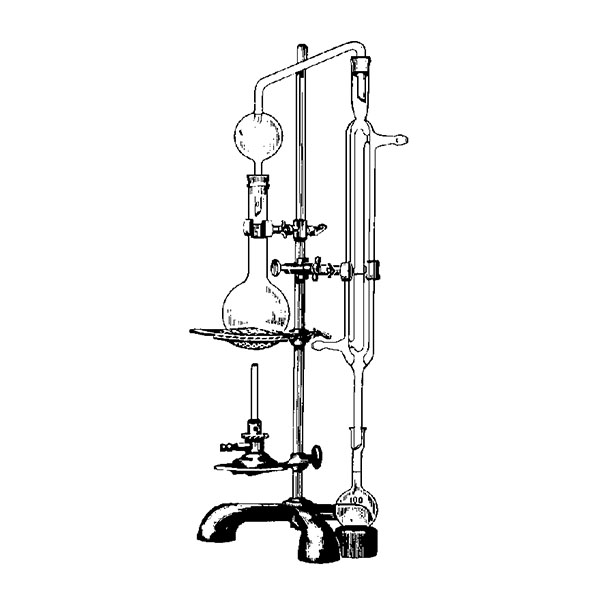
Laboratory distiller
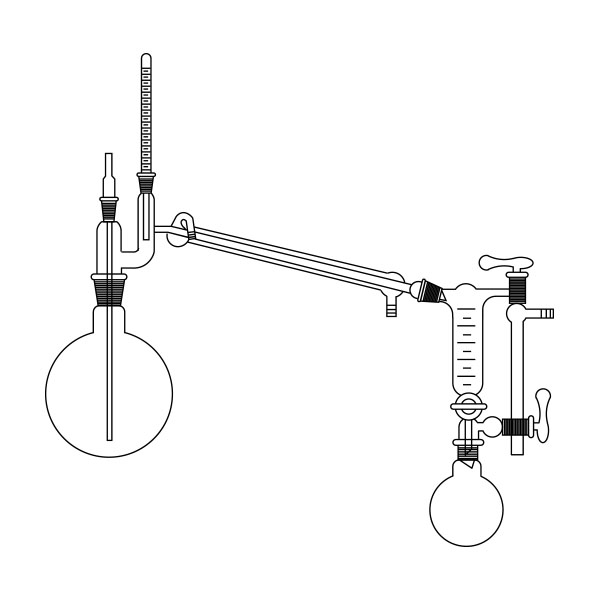
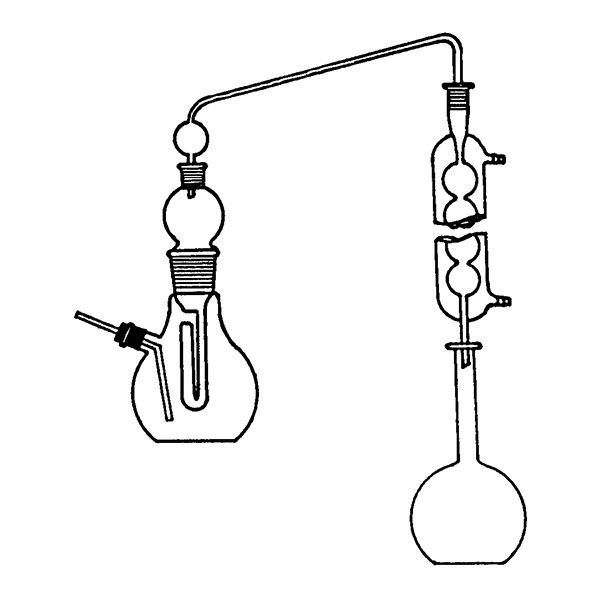
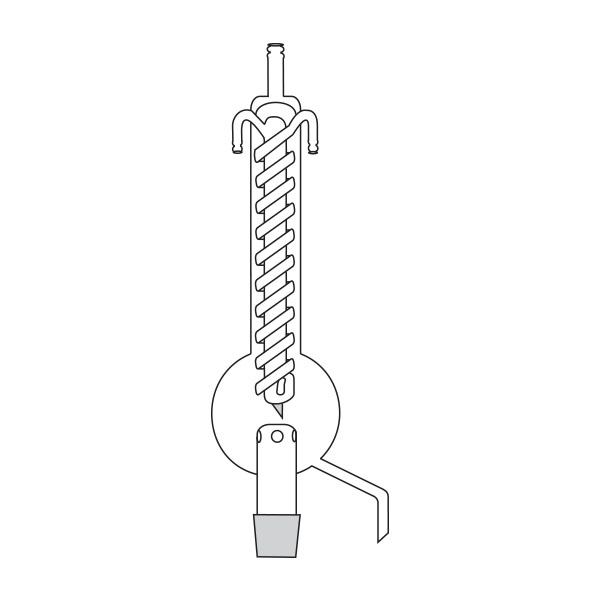
The laboratory distiller has equipment with specific characteristics. The distiller is generally combined with a vessel or flask, the task of which is to contain the liquid to be heated. A condensation column, often a Vigreux column, in which the vapours are cooled to a liquid state. A Claisen tube, which has the task of conveying the vapours to the condenser, where they will then be condensed. A container, in which the distilled liquid is finally collected.
.

Laboratory Distillation Techniques
laboratory distillation
The steam is immediately channelled to the condenser. As a result, the distillate is not pure, but of the same composition as the vapour.
Fractional distillation
It is used to separate different components into separate distilled fractions. It is obtained through repeated vaporisation and condensation processes. The principle behind this type is the different volatilities of the mixture components. The distillation column used for fractional distillation is called a 'fractionation column'.
Vapour-current distillation
Used for thermolabile components. The addition of steam or water lowers the boiling point of substances.
The addition of steam or water lowers the boiling point of substances.
Vacuum Distillation
This differs from the classic type in that it is carried out under vacuum. It is used when the substance to be distilled has a high boiling temperature. In fact, the principle is exploited whereby the liquid reaches boiling point when its pressure equals atmospheric pressure.
It is used when the substance to be distilled has a high boiling temperature.
Those interested in learning more about this topic can find further information about the water distiller or at distiller-for-essential-oils.
 Azeotropic distillation
Azeotropic distillation
Azeotropic distillation is a method of separating mixtures according to their differences in volatility in a boiling liquid mixture. An azeotrope (a mixture of two or more vapour-producing liquids with the same ratio of components as the original mixture) cannot be separated by normal distillation, so a third additional component is added to the mixture. This has the effect of changing the volatility of one of the liquids in the azeotrope to a greater extent than the other, allowing it to be separated.
 Industrial applications: oil distillation
Industrial applications: oil distillation
One of the applications of distillation that is carried out outside a chemical laboratory is that of petroleum. Petroleum is a complex mixture of organic liquids that occurs naturally in the ground and was formed millions of years ago. Crude oil varies in colour and composition, from a pale yellow low-viscosity liquid to a black colour of higher viscosity.
The distillation of crude oil is the first step in the refining process. It is carried out by heating the crude oil to 350° and introducing it into the lower part of the column of the appropriate equipment, at ambient pressure. The distillation column consists of a series of plates, characterised by a particular bell-shaped structure. These have a decreasing temperature towards the top and on each of them condense the components that have a boiling temperature similar to that of the plate. The fraction that has not condensed and reaches the bottom of the column is called 'bitumen'.



 The distillation is a technique known since antiquity and is carried out using the distiller. There are documents indicating its use by Egyptian peoples in 4000 BC, for the distillation of alcoholic beverages. Subsequently, the use of the distiller in the laboratory was already known in the Middle Ages.
The distillation is a technique known since antiquity and is carried out using the distiller. There are documents indicating its use by Egyptian peoples in 4000 BC, for the distillation of alcoholic beverages. Subsequently, the use of the distiller in the laboratory was already known in the Middle Ages.
 Azeotropic distillation
Azeotropic distillation Industrial applications: oil distillation
Industrial applications: oil distillation