The density of a material, or density, is defined as the ratio of the mass to the volume of that material. In a chemical laboratory, calculating the density of a sample is possible through the use of various equipment.
Density measurement with pycnometers
.
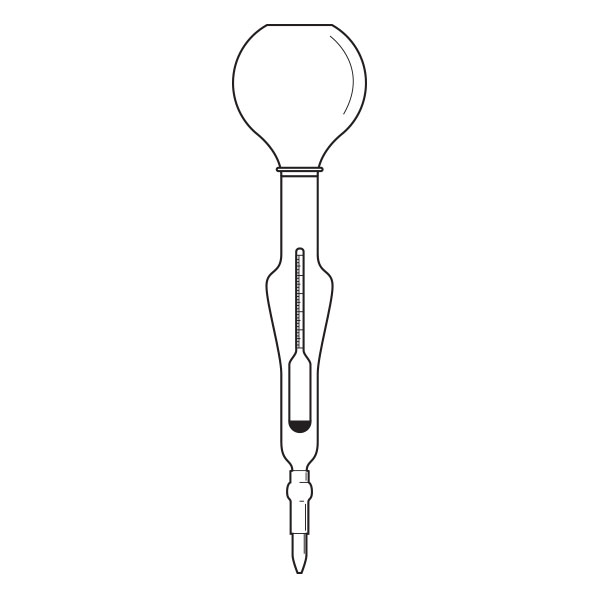
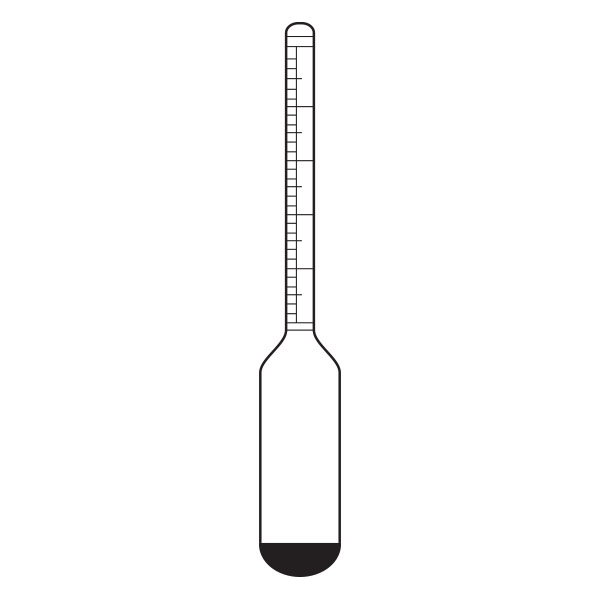
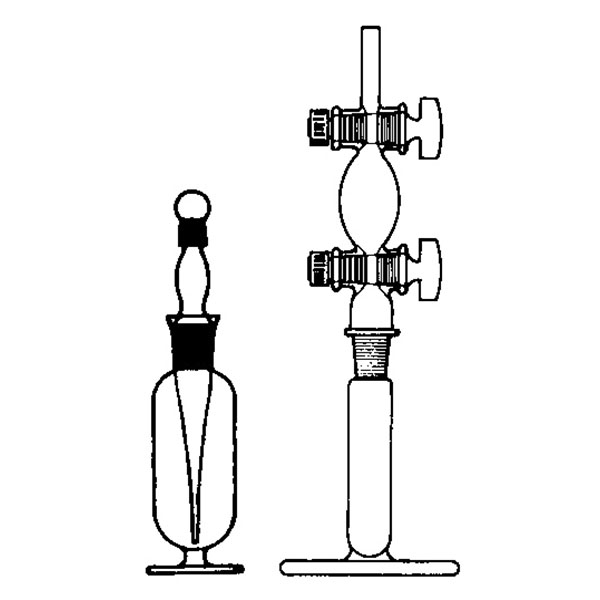
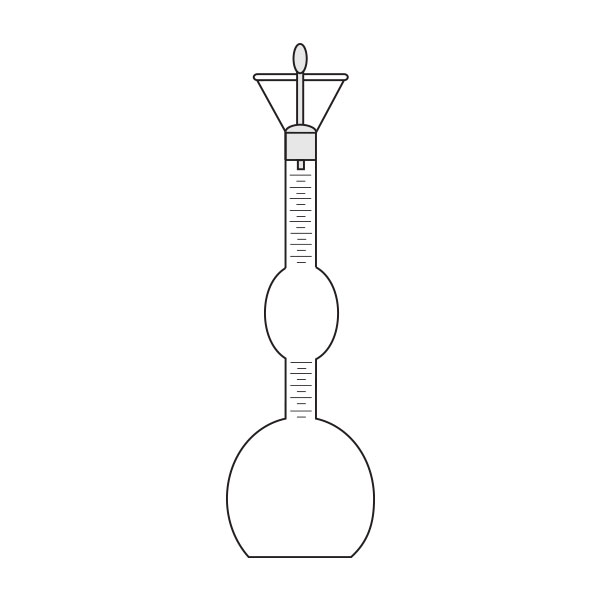
The pycnometer is one of the instruments with which the density of a liquid sample can be calculated. It is a glass container, sealed with a capillary tube and a reference. This has the purpose of defining the level of the liquid inside the pycnometer and allowing air bubbles to escape from the instrument.
The pycnometer allows an accurate calculation of the density of a liquid in relation to a sample fluid, such as water or mercury, by means of an analytical balance.
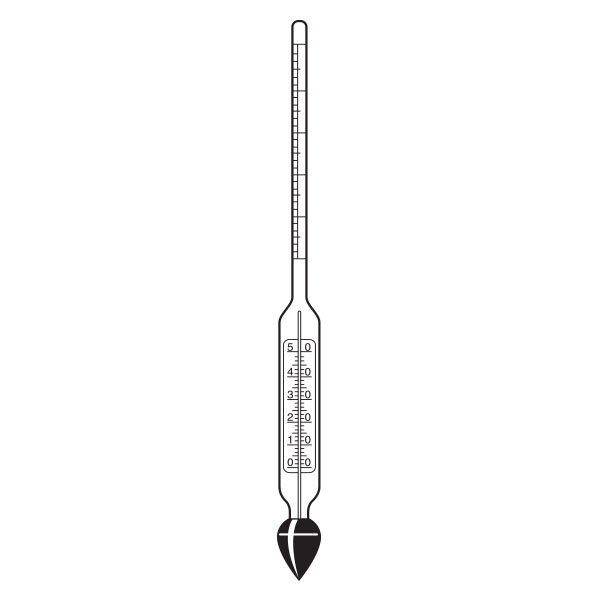
There are several types of pycnometer, including Gay Lussac's, characterised by its pear shape and capillary-hole cap, and Hubbard's, with its cylindrical or conical shape. The latter is often used to calculate the density of bitumen or heavy crude oils.
Measuring density with density meters
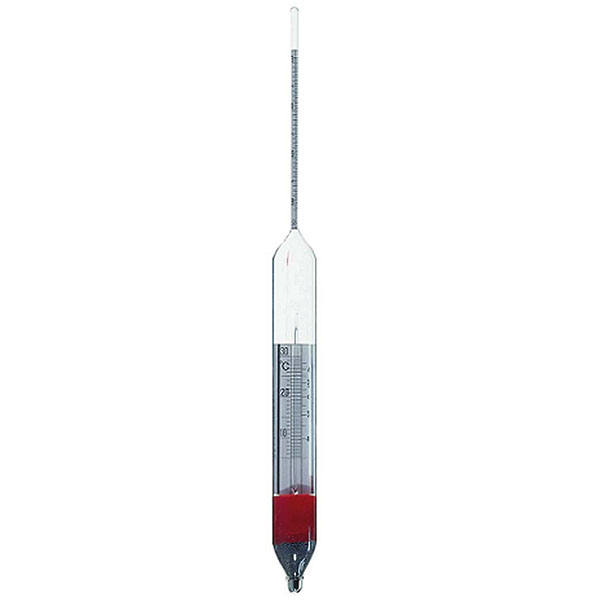
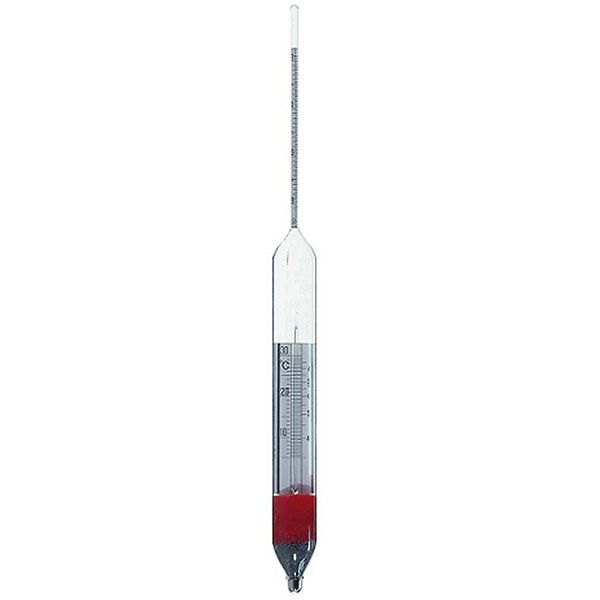
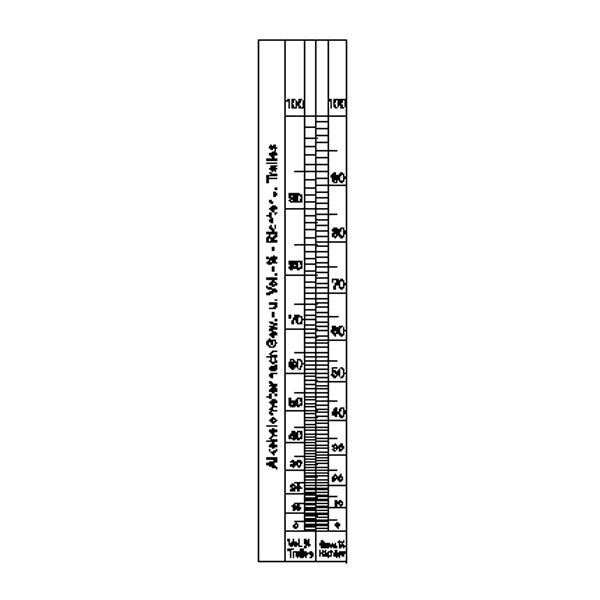
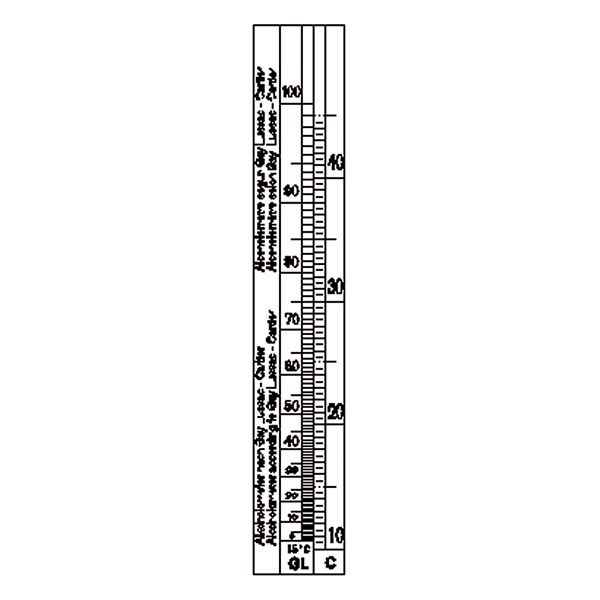
An additional instrument for measuring density is the densimeter, also referred to as an aerometer. The densimeter is used to measure the density of liquids, and is based on the principle of Archimedes' buoyancy. It is immersed as a float in the liquid under study, and its specific gravity is measured by taking into account two factors. The first is its upper section, which emerges from the liquid and acts as a measuring scale. While the second is the weights that contribute to making the densimeter float.
The densimeter is also divided into two main types: constant weight and constant volume.
Other instruments
There are also other instruments useful for measuring density. For example, Babo's must meter is used for measuring the percentage of sugars contained in the must. Consequently, this instrument allows the probable alcoholic degree of the wine to be measured.
Another instrument is the saccharimeter, used in measuring the concentration of sucrose in a solution. Finally, the volumenometer is an instrument that allows the volume of solids that cannot be immersed in liquid substances to be calculated. The calculation is done by expanding air inside it, under known pressure conditions. The measurement is carried out twice, with and without the solid inside the volumeter. A subsequent calculation will define the volume of the solid.
.